NOTE: This article can also be downloaded and shared (without the style of this website): Wiki World Order’s Turtles Book Review [PDF]
Video Version: Odysee (2 minute trailer)
Audio Only: MP3

For complicated narratives and debates, I hunger for the strongest arguments and evidence from all sides. Fortunately, I was gifted an impressive book from 2022 titled “Turtles All The Way Down: Vaccine Science and Myth.” This post contains some of the book’s most potent claims, framings, summaries, and questions on vaccine sub-topics.
In the information age, asking the right questions is critical, and this book includes questions that people can ask their doctor. Asking most of these questions to a doctor during your regular visit might incite an adverse reaction. These questions might not be safe nor effective for your personal pediatric situation. But I have copied these questions, and I encourage vaccine proponents and skeptics alike to search for and share the answers that you find.
I am not giving medical advice, and this is also not legal advice, nor financial advice.
Do you support using governments to pressure your neighbors into taking vaccines? Then please steelman some of this book’s key questions and be sure to share answers with references.
There are many footnotes printed within this book, but it’s hundreds of references can be downloaded online here:
tinyurl.com/TurtlesBookEngRef
In the book’s forward, Mary Holland, J.D., president and general counsel at Children’s Health Defense (CHD), writes that this book…
“was first published in Israel in early 2019. Later that year, […] a mainstream medical journal published a positive review of it. […] To the dismay of the Israeli medical establishment, [the senior academics] ‘found the book to be well written, serious, scientific and important’, offering ‘a comprehensive view of the issue.'”
page 18
“One academic, Daniel Mishori, Ph.D., a senior faculty member specializing in ethics and philosophy at the Department of Environmental Science at Tel Aviv University, was so disturbed by the lack of discussion of the book’s arguments that he offered a cash prize ($4,000 donated to the hospital ward of choice) to anyone who could refute them. Since Turtles is over 500 pages and contains more than 1,200 references, Mishori declared he would settle for a proper rebuttal to the harsh conclusions drawn in the first chapter of the book. To this day no one has been able to meet his ‘Turtles challenge.'”
Page 19
“Another good reason the authors chose anonymity, and perhaps the more important one from your perspective as a reader, is to ‘immunize’ the book against ad hominem attacks, a favorite tactic employed by the pharmaceutical industry and the medical establishment. […] However, this ‘shoot the messenger’ tactic only works if there is someone to shoot.”
Page 20
Part I: Vaccine Safety
Chapter 1: Vaccine Clinical Trials
(50 pages, 70 references)
The first chapter explains basic definitions and processes for randomized controlled trials (RCTs). It then breaks down key flaws and distortions to the term “placebo”. This entire chapter is freely available online, and I would strongly encourage everyone to read it:
tinyurl.com/TurtlesBookChap1Eng
“Giving the control group an active substance in an RCT intended to test safety would be a bad design decision, then. Yet this is exactly how new vaccine Phase 3 trials are performed: Instead of a placebo, the control group receives a different vaccine, which is certain to cause its own adverse events and can in no way be deemed a neutral substance.
Page 54
[…]
This deliberate distortion of the placebo concept in clinical trials of new vaccines is so prevalent that researchers and vaccine package inserts frequently refer to the bioactive compound given to a control group as ‘placebo’, even when it’s clear it is another vaccine or a similar bioactive compound, which in itself is not safety-neutral.15 Falsely using the term ‘placebo’ allows researchers to conclude that the new compound ‘was proven safe’ even though the substance the control group received was decidedly not a placebo.
[…]
No logical explanation can be found for the ubiquitous practice of administering bioactive compounds to control groups in trials of new vaccines other than a desire to conceal the true rate of adverse effects of the vaccine.”
Chapter 1, Reference 15: VAQTA Package Insert
Discussing a case study of the Diphtheria-Tetanus-acellular-Pertussis (DTaP) vaccines by GlaxoSmithKline (GSK) and Sanofi Pasteur…
“To summarize, the safety of GSK’s 5-in-1 and 4-in-1 vaccines was tested against the triple vaccine (DTaP), which was tested against the older generation vaccine (DTP), whose safety was never tested in an RCT with a placebo control group. A turtle standing on the back of a turtle, standing on the back of yet another turtle — all the way down.
Page 57
[…]
Daptacel, Sanofi’s triple DTaP vaccine, underwent four clinical trials during its licensing process. All of the trials were randomized and controlled, and in all of them, the control group received different combinations of DTaP or DTP vaccines, sometimes concurrently with other vaccines as well.29
[…]
since the rates for the new-generation-vaccine (DTaP) groups were similar to that of the old-generation-vaccine (DTP) groups, the new vaccines received the green light.
The bottom line is that none of the many products in either of the DTaP vaccine family lines routinely administered in the US has been tested for safety in a clinical trial with a placebo-controlled group.”
Chapter 1, Reference 29: DAPTACEL Package Insert
This book goes on to describe the ‘safety turtles’ belonging to vaccines on the childhood vaccine schedule of the Centers for Disease Control and Prevention (CDC).
Table 1: The control group in Phase 3 clinical trials of CDC’s routine childhood vaccines“Childhood Vaccine Clinical Trials: A Summary
Page 72 (table Reproduced Below)
The table below summarizes the safety testing performed in Phase 3 clinical trials for the vaccines included in the CDC-recommended childhood vaccination program.”
| Disease | Vaccine | The Control Group in Phase III Clinical Trials |
|---|---|---|
| Diphtheria-Tetanus-Pertussis (with and without Polio, Hepatitis B and Hib) | Pediarix (Diphtheria-Tetanus-Acellular-Pertussis-Hepatitis B-Polio) | Control groups in the trials received either the Infanrix vaccine along with hepatitis B, Hib, and polio vaccines, or other, unspecified vaccines. No control group received a placebo. |
| Kinrix (Diphtheria-Tetanus-Acellular-Pertussis-Polio) | In the only trial specifically described in the package insert the control group received the Infanrix and polio vaccines. The package insert doesn’t mention any trial involving a placebo control group. | |
| Infanrix (Diphtheria-Tetanus-Acellular-Pertussis) | Tested against a control group that received the DTP vaccine or no control group. | |
| DTP (Diphtheria-Tetanus-Pertussis) | The vaccine was developed in the 1930s and has never been tested in an RCT against a control group receiving a real placebo. | |
| Pentacel (Diphtheria-Tetanus-Acellular-Pertussis-Polio-Hib) | The control groups in 3 of the 4 trials received an assortment of different vaccines. The 4th trial’s control group may have received no vaccines; however, its safety data is not presented in the package insert. | |
| Quadracel (Diphtheria-Tetanus-Acellular-Pertussis-Polio) | The control group in the trial received the Daptacel and polio vaccines. | |
| Daptacel (Diphtheria-Tetanus-Acellular-Pertussis) | The control groups in the trials received other vaccines. | |
| Haemophilus Influenzae Type B | Hiberix | The only blinded RCT had two control groups receiving another Hib vaccine or a DTaP-polio-Hib, along with several other vaccines. |
| ActHIB | Control groups received either the DTP vaccine or other combination DTaP-based vaccines. | |
| PedvaxHIB | Most of the control group subjects received DTP and OPV vaccines along with a “placebo” whose ingredients were not specified. | |
| Polio | IPOL | This package insert does not mention any RCT performed for the vaccine. |
| Pneumococcal Disease | Prevnar-13 | Tested against a control group receiving Prevnar (older-generation vaccine). |
| Prevnar | Tested against a control group that received an experimental meningococcal vaccine. | |
| Hepatitis B | Engerix | Its side effect rate was compared to that of a previous generation product (plasma vaccine). |
| Twinrix | Tested in clinical trials against a control group that received separate hepatitis A and B vaccines. | |
| Recombivax HB | The package insert does not mention any safety RCT performed in infants. | |
| Hepatitis A | Havrix | The control group in the main trial received the hepatitis B vaccine. In three other trials, the control group received several other vaccines (MMR, varicella vaccine, and more). |
| Vaqta | In one trial, there was no control group (according to another document, the control group received a compound that included aluminum and thimerosal), and in the second trial the vaccine was given concurrently with other vaccines and without a control group. | |
| Measles, Mumps, Rubella, Varicella (Chickenpox) | ProQuad (Measles, Mumps, Rubella, Varicella) | Safety was tested in several randomized clinical trials, most of which were not blinded. None of the trials contained a control group receiving only a placebo. |
| MMR II (Measles, Mumps, Rubella) | Tested in eight small unblinded clinical trials. All of the trials had one or more control groups receiving either the predecessor MMR vaccine, a measles-rubella (MR) vaccine, or a single-dose of the rubella vaccine. | |
| MMR (Measles, Mumps, Rubella) | Tested in several small to medium unblinded and partially randomized trials. The control groups totaled about 1/10 the number of subjects in the trial groups and received no injection. | |
| Varivax (Varicella) | In one RCT the “placebo” given to the control group was actually the test vaccine from which the viral component was removed. Another trial compared two different formulations of the vaccine. | |
| Rotavirus | RotaTeq | The control group in the trial probably received the vaccine-sans-antigen compound (the description of the control compound was intentionally deleted from FDA licensing documents.) |
| Rotarix | The control group in the trial received the vaccine-sans-antigen compound. |
“The claim made in this chapter is not that vaccines in general are never tested against placebo in their pre-licensure process. Rather, it is childhood vaccines recommended by the CDC that were never tested against a placebo.”
Page 78
“In short, the vast majority of physicians and researchers are completely unaware of the manner in which vaccine safety trials are designed and conducted and the methodological flaws inherent in that process.
Page 81
[…]
Summary
Vaccines, as opposed to drugs, are given to healthy babies and thus must meet a particularly high safety standard. Clinical trials of new vaccines must be impeccably designed and performed, thereby providing high-quality, reliable data about the products’ efficacy, and more importantly, about their safety. Anything less is socially and morally unacceptable.
[…]
As we have seen in this chapter, vaccine trials are designed and performed in such a way as to ensure that the true extent of adverse events is hidden from the public. There is not a single vaccine in the US routine childhood vaccination program whose true rate of adverse events is known.
[…]
Current vaccine clinical trial methodology completely invalidates the claims that vaccines are safe and that they are thoroughly and rigorously tested.”
“Ask your doctor:
Page 83
Was the vaccine that you are recommending tested in a pre-licensure clinical trial with a (real) placebo control group? If not, how do you (or anyone else, for that matter) calculate its true rate of adverse events?
Is it morally acceptable to conduct a clinical trial in infants for a new vaccine, where the ‘control group’ receives an untested compound, i.e., the vaccine-sans-antigen, which is likely to cause irreversible side effects and has no potential benefit?“
Reminder: This potent first chapter is available online for free.
Debunking Attempts: What About Gardasil?
After finishing the Turtles book, I searched for rebuttals and debunkings. I found one criticism by Dr. Frank Han that the authors didn’t bring up the HPV vaccine which is part of the childhood schedule. He claims that its package insert says that it was tested against a placebo.
Indeed, the book’s first chapter only includes a footnote on HPV, so perhaps this is a valid criticism. While discussing the rotavirus vaccine trials, the Chapter 1 footnote on page 71 says: “bb A similar, though not identical, design was employed in the pre-licensure trials of the human papillomavirus (HPV) vaccine Gardasil.” The book describes the Rotavirus clinical trials over three pages in the chapter, and summarize it in the table above. The Gardasil insert says:
“[…] 6.1 Clinical Trials Experience
Gardasil package insert, page 4
In 7 clinical trials (5 Amorphous Aluminum Hydroxyphosphate Sulfate [AAHS]-controlled, 1 saline placebo-controlled, and 1 uncontrolled), 18,083 individuals were administered GARDASIL or AAHS control or saline placebo on the day of enrollment, and approximately 2 and 6 months thereafter, and safety was evaluated using vaccination report cards (VRC)-aided surveillance for 14 days after each injection […]”
So only one of seven trials was controlled with a saline placebo. I looked at the injection-site adverse reaction tables (Tables 3 and 4) to see how many participants got all three doses. 7,644 got Gardasil, 5,094 got the AAHS ‘control’, and only 559 got the Saline Placebo.
I don’t [yet] have the expertise to know how professionals might describe the statistical power of their one saline placebo trial. But to me, this ‘safety’ control group in the hundreds is far too small to then jump to 100s of millions of doses.
The injection-site adverse events data tables do show many more adverse events in the AAHS control arm than the saline placebo arm. But these reactions are generally not severe or life-threatening:
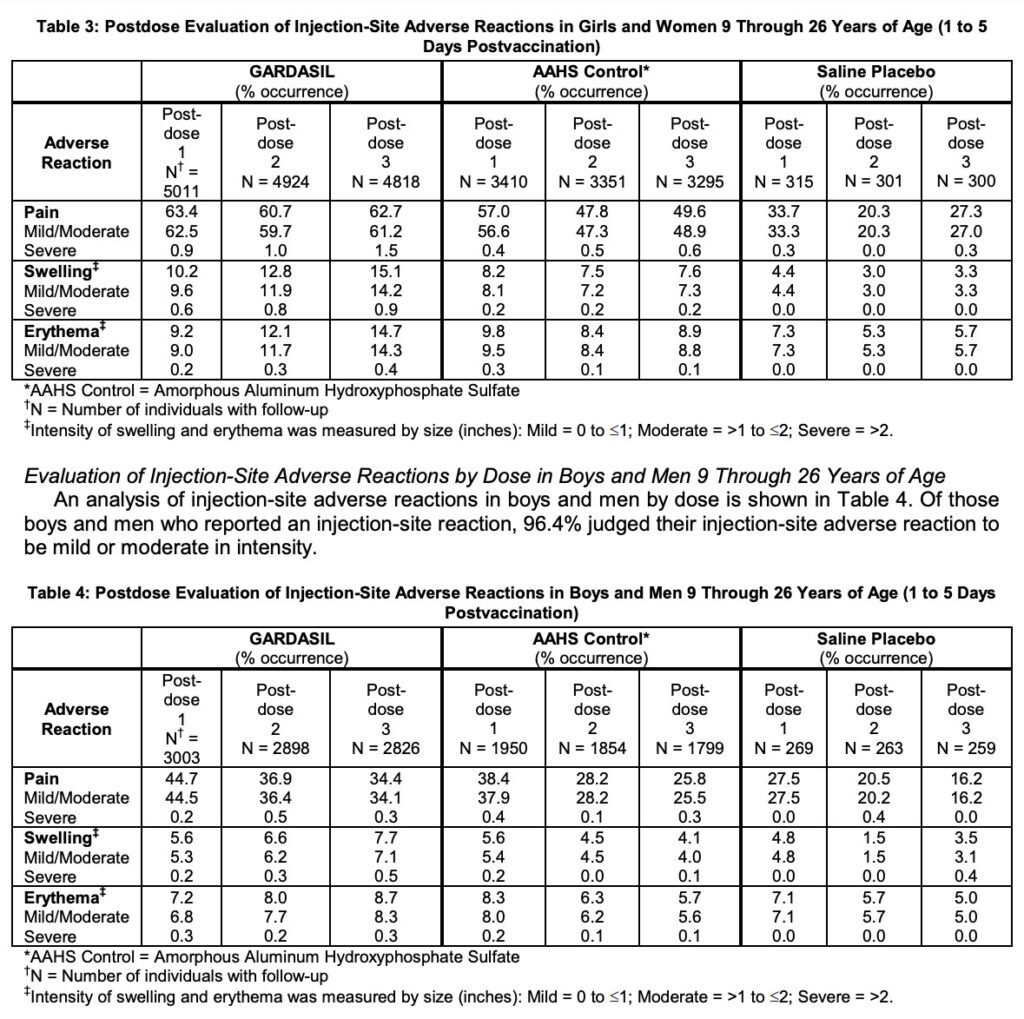
The next data tables cover Systemic Adverse Reactions and Systemic Autoimmune Disorders. But here, the authors merge the AAHS Control with the Saline Placebo, hiding any more serious safety signals. There is almost no other data provided on how Gardasil or the AAHS Control compares to the Saline Placebo.
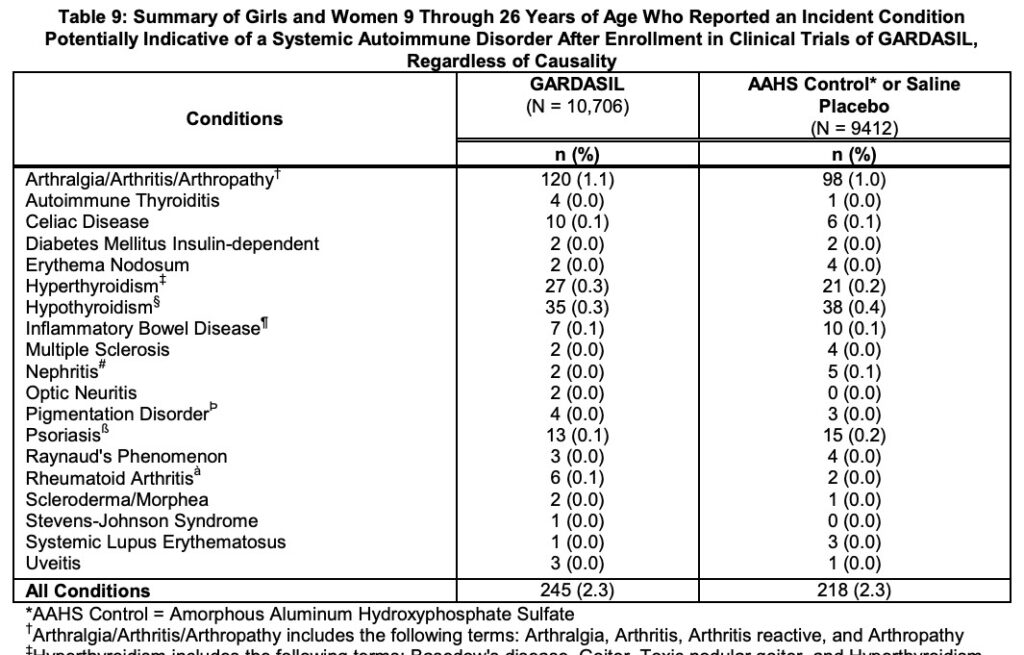
So it seems that the Gardasil trials did include one saline placebo control arm, but it was almost 1/10 the size of the vaccine-sans-antigen arms. And Gardasil did not share the most relevant safety data for the placebo arm separately.
Dr. Han also cites the Gardasil-9 package insert as a counter example for the book’s claim. It does say they ran one trial with a saline placebo looking for adverse events within 14 days (GARDASIL-9 N=608, Saline Placebo N=305). But this [side-trial] only included girls and women (age 12-26) “who had previously been vaccinated with three doses of GARDASIL”.
The raw data table also does show increases in most adverse events for GARDASIL-9 compared to the saline placebo. And in the Systemic Autoimmune Disorders section, they lump together historical clinical trials of the saline placebo with the old GARDASIL and the AAHS control. So once again, potentially useful saline placebo safety data was not included in the package insert.
So at worst, maybe the book’s claim should revised to be something like… “childhood vaccines recommended by the CDC were never tested against placebo with published safety data.”
The Turtles book does not cover COVID-19 vaccines, which are unique in their technology and Trump’s Operation Warp Speed. I did not detect any significant blows landed in Dr. Han’s other four articles. He tended to confirm the book’s key claims, while using lots of COVID-19 examples, other straw men arguments, and group think rhetoric. Most of his counterarguments were also prophylactically refuted in the book.
Debunking Attempts: Polio & Hep B Vaccines?
In my searches for rebuttals and debunkings of this book, I found one more attempt by a legal academic, Professor Dorit Rubinstein Reiss. She claims polio is a counter-example, and that “the authors completely ignore the large placebo-based trials conducted for the Salk vaccine – whose formula is still the one used in the United States – in the 1950s.”
Reading through the study, “An evaluation of the 1954 poliomyelitis vaccine trials: summary report” by Francis T, et al. (1955), “One half would receive vaccine; the other matching half, serving as strict controls, would receive a solution of similar appearance which should have no influence on immunity to poliomyelitis.” I had trouble concluding what that placebo was, other than “consisting of harmless culture media.”
The book doesn’t go deep into polio in Chapter 1, saving its complex history for Chapter 10. In Chapter 1’s summary table, the book just cites the IPOL package insert. This package insert does not include the word ‘placebo’. The short ‘WARNINGS’ section there says, “Systemic adverse reactions reported in infants receiving IPV concomitantly at separate sites or combined with DTP have been similar to those associated with administration of DTP alone. (11)” But that footnote 11 refers the reader to “Unpublished data available from Sanofi Pasteur SA.”
Professor Reiss then discusses the Hepatitis B vaccine, claiming that, “as with Hib vaccines and polio vaccines, the book discussion here does not start with the first vaccine. The first hepatitis B vaccine was a Merck vaccine – a plasma vaccine – in 1981. This plasma vaccine was tested against a placebo. The second hepatitis B vaccine, by Merck, was licensed in 1986. This vaccine was licensed in 1989.”
Her link for the 1981 plasma vaccine says, “The vaccine and placebo were packaged in visually indistinguishable 1-ml vials. One dose of the vaccine contained 40 pg of HBsAg protein, subtype ad, in an alum adjuvant (Lot 751). The placebo consisted of alum alone in the vaccine diluent.” This is not an inert placebo, confirming the book’s claims.
The full 1986 package insert only mentions ‘placebo’ in footnote 6 which cites, “Efficacy of Hepatitis B Immune Globulin for Prevention of Perinatal Transmission of the Hepatitis B Virus Carrier State: Final Report of a Randomized Double Blind, Placebo-Controlled Trial” (Beasley, R.P., et al, 1983). And this referenced article does not describe the placebo used. The 1989 package insert also does not mention ‘placebo’ at all.
So thus far, I’m not seeing solid counter-evidence for the book’s claim that: polio and Hep B vaccine trials did not test it against an inert placebo.
Debunking Attempts: Saline Placebos Would Be Unethical
Before his first Gardasil critique, Dr. Han also referenced a broader article on placebo trial ethics by Dr. David Gorski. Most of Dr. Gorski’s arguments explain, rationalize, and defend the current industry standards. He explains their ethical framework and “clinical equipoise.” The establishment claims that clinical trials are unethical if they withhold “standard of care” treatments already in use.
Many examples used to explain ‘clinical equipoise’ involve treatments for patients who already have life-threatening medical conditions. But an unvaccinated infant does not inherently have a life-threatening medical condition. Turtles describes almost all of the diseases in the childhood vaccine schedule as having ‘moderate’ to ‘mild’ severity (Chapter 9). So the diseases that infants might contract later have low probabilities of life-threatening complications. This is a dramatically different starting point than patients who are already on their death bed.
Furthermore, “Clinical equipoise is defined as the genuine uncertainty within the scientific and medical community as to which of two interventions is clinically superior (Freedman, 1987b).” But the industry ignores, de-platforms, or excommunicates experts who cast doubt on vaccine safety science. Thus, the establishment orthodoxy denies any ‘genuine uncertainty,’ and self-validate their ethical framework.
But what if this scientific process were fair and transparent? Then there would be plenty of ‘genuine uncertainty’ to ethically justify pre-licensure clinical vaccine trials against real placebos.
The Turtles book describes the option of three-arm clinical trials:
“In order to determine the true rate of adverse events of a new generation vaccine, a three-arm trial must be conducted, combining the two methods described above. In this kind of trial, subjects would be randomly allocated into three groups, one trial and two controls: The trial group would receive the new generation vaccine, the first control group would receive the current vaccine, and the second control group would receive an inert placebo. This trial design is considered to be of excellent quality, as it measures both the absolute rate of adverse events (comparing the new vaccine to the placebo) and the relative rate (comparing the new vaccine to the current vaccine).8“
Page 45
Chapter 1: Reference 8: ICH Harmonised Tripartite Guideline Choice Of Control Group And Related Issues In Clinical Trials E10 (P 12 (18))
And the Turtles book also briefly responds to the establishment claim that “It is unethical not to give the control group another vaccine”:
“In fact, the opposite is true: It is unethical not to conduct at least one trial from which one can reliably estimate the rate of adverse events before a vaccine is licensed and widely used.
Page 76
As reviewed in this chapter, medical ethics guidelines permit the administration of a placebo to a control group in a clinical trial of a completely new vaccine and to a control group in a three-arm trial of a next-generation vaccine.”
Dr. Gorski writes, “I will instead argue that a much better way of looking at a placebo control is that it should be ‘inert’ with respect to the outcomes being studied in the RCT.” So to most accurately calculate efficacy, this perspective requires an “immunologically inert” placebo that could not impact the disease in question. If the industry and regulators shared the safety concerns of millions of parents, then placebos might be “biologically inert.” Such safety-focused placebos would be closer to the CDC’s definition for ‘placebo’ as “A substance or treatment that has no effect on living beings.”
But phase 3 trials are supposed to study safety, efficacy, and dosing. And Dr. Gorski defends placebo selections for efficacy-based outcomes instead of safety-based outcomes. So logically, this is an admission that phase 3 vaccine trials are not designed to test for safety. In total, as I read it, Dr. Gorski’s article does nothing to refute the claims in the Turtles book.
If placebos cannot be designed to test for both efficacy and safety, then large-scale efficacy and safety trials must need to be split up. This could simply look like the three-arm trials that the Turtles book suggests. And I agree that the safety arm of the trials should not be 1/10 the size of the other arms. The safety arm must also be scaled to identify rare adverse events before a vaccine given to 100s of millions of people.
The establishment also discusses ‘personal equipoise.’ But they seem to ignore the obvious option of letting parents opt into the risks of a real placebo trial with fully informed consent of all current knowns and unknowns of each disease and each vaccine. Surely that must be ethical and legal, but they never mention this possibility.
Health authorities have been so panicked about the increasing millions of vaccine hesitant parents that they conduct studies on communicating vaccine safety. Therefore there are likely plenty of candidates willing to provide informed consent to real placebo trials.
Contrary to most scientific paradigms, and with great hubris, vaccine evangelists seem to believe their paradigm will still be standing tall a century or two from now. They also seem to have similar certainty in their ‘medical ethics’ paradigm. They seem astonished that anyone would question the dogma, hold informed but contradictory opinions, or perceive different ethical priorities.
This book was published by anonymous authors. But it is interesting how each these debunking articles still try to shoot the messenger (the classic ad hominem logical fallacy).
Reading through counter-arguments for Chapter 1, the facts seem generally agreed to. But the interpretations and ethical rationale diverge greatly. There is a giant gap between how industry~supported scientists and physicians evaluate the certainty of safety data, compared to those who are industry~silenced.
The establishment ethics rely on the assumption of safety, an echo chamber, and circular logic. They claim the existing vaccines have already been proven safe and effective beyond genuine uncertainty. And this validates their ethical paradigm that designs vaccines’ clinical trials.
But informed and ‘skeptical’ scientists and parents are challenging the certainty of existing safety science. Skeptics have been complaining that there is inadequate data to prove a net benefit. Based on these different conclusions from these vaccines’ safety data, skeptics disagree with the industry’s ethical paradigm. We demand more safety-focused placebo data — orders of magnitude more.
Chapter 2: The Science of Vaccine Adverse Events: A Missing Link and an Empty Toolbox
(20 pages, 34 references)
The previous chapter reviewed the pre-licensure process for childhood vaccines. Then Chapter 2 focuses on post-licensure safety reporting and studies.
“Part of the American National Academy of Sciences, the Institute of Medicine (IOM)f is a nonprofit, non-governmental organization that serves as an advisory body to the nation on medical and health issues. Its role is to provide objective and reliable information and advice to policymakers, the medical profession, and the public.
Page 90
[…]
In 2011, in response to a request submitted by the US Department of Health in 2009, the IOM issued a special report on vaccine adverse events.6 The IOM was asked to convene an expert committee ‘to review the epidemiological, clinical, and biological evidence regarding adverse health events associated with specific vaccines covered by the [vaccine program]’ and to state its opinion on ‘the evidence bearing on causality, and the evidence regarding the biological mechanisms that underlie specific theories for how a specific vaccine is related to a specific adverse event.’7
[…]
It took the committee two full years of demanding work to complete the report, during which it carried out a thorough investigation into the available evidence, including epidemiological (statistical), clinical (medical studies in humans), and biological (animal and in vitro) studies. The committee’s conclusion was that there were only a handful of adverse events of specific vaccines for which a casual link between the event and the vaccine had been established. Of the 158 adverse event and vaccine combinations examined by the committee,8 h a causal link was confirmed for only 14 of them and suggested for 4 others (‘the evidence favors acceptance’).9
[…]
Almost half, 6 of the 14, were labeled ‘anaphylaxis’, an acute, immediate, and often life-threatening allergic reaction, which the committee associated with 6 different vaccines. […] the link between vaccination and outcome was virtually irrefutable for 12 of the 14 adverse events that the committee confirmed were causally linked to vaccination.”
Chapter 2: Footnote f: In 2015, the IOM changed its name to the National Academy of Medicine (NAM)
Chapter 2: Reference 6: IOM, 2011: Adverse Effects of Vaccines: Evidence and Causality
Chapter 2: Reference 7: Adverse Effects of Vaccines: Evidence and Causality (P 30 (59))
Chapter 2: Reference 8: IOM 2011 (P IX (10))
Chapter 2: Footnote h: The committee examined 158 pairs of adverse-events/vaccines. Some of the adverse events were investigated with relation to several vaccines. Thirty different adverse events were examined for the MMR vaccine; 15 for varicella; 27 for influenza; 8 for hepatitis A; 27 for hepatitis B; 13 for human papillomavirus; 9 for meningococcal; and 3 related to the act of injection itself.
Chapter 2: Reference 9: IOM 2011 (P IX (10))
“So the majority of the scientific literature regarding disease mechanisms consisted of superficial case studies, and the committee determined that only a handful of papers met the criteria of basic scientific research — studies that looked for causal links between vaccines and medical conditions through in-depth investigation of biological mechanisms. It is no wonder, then, that the committee could not confirm or deny a causal link to vaccination for over 85% of the adverse events examined.
Page 94
[…]
The shortage of applicable scientific research on adverse events documented in its own report should have prompted the IOM committee to sound a long and loud alarm. […] Yet, instead of sounding the warranted alarm, the committee chose to give the ‘all-clear’. The report’s conclusions don’t convey any sense of urgency, nor does the report call for health authorities to take any immediate action. While the report does not state it explicitly, news articles following the report’s publication conveyed the committee chairperson’s implicit message that All is fine: Only a handful of adverse events have been proven to be linked to vaccines, and even those are mild and rare.
The IOM committee members know that ‘the absence of evidence is not evidence of absence’27 but went ahead and acquitted vaccines anyway based on a lack-of-evidence argument.”
Chapter 2: Reference 27: IOM 2011 (P 14 (43))
When researching these types of tough topics, I find it critical to review official reports like the IOM 2011. It is probably worth reading through more, if you’re curious.
“Ask you doctor:
Page 104
If our child experienced a health problem following vaccination, what medical tests are at your disposal to decide whether the condition was actually caused by the vaccine?
We fear that our child could be adversely affected by a particular vaccine. What medical tests can you perform in order to determine whether or not she is at high risk of being injured by that vaccine?“
Chapter 3: Deficient by Design: Vaccine Adverse Event Reporting Systems
(22 pages, 50 references)
“Vaccine adverse event reporting systems around the world have similar modes of operation and functionality. Therefore, we will focus on the world’s leading system: the US Vaccine Adverse Event Reporting System commonly referred to as VAERS.
Page 107
The VAERS system, jointly managed by the CDC and FDA, was established under the National Childhood Vaccine Injury Act of 1986 and began operation in 1990.3“
Chapter 3, Reference 3: Understanding vaccine safety information from the Vaccine Adverse Event Reporting System (Varricchio 2004)
“While VAERS, with its grossly underreported, incomplete, non-randomized, and uncontrolled data, cannot contribute meaningfully to vaccine safety, it is ideal for those who might wish to obscure any links between vaccines and adverse events.”
Page 117
“This suggests that VAERS and similar systems were intentionally designed to serve as ‘window dressing’, that is, to provide a mere semblance of vaccine safety monitoring rather than the real thing. In practice, these reporting systems constitute another link in a chain, which began in clinical trials, aimed at preventing the truth about the alarming rate of vaccine adverse events from gaining public attention.”
Page 119
In addition to VAERS, their is also the Vaccine Safety Datalink (VSD) system:
“The VSD system is not an adverse event reporting system and is therefore not covered in this chapter. It is a CDC-run information network that pools data from several major US healthcare providers’ computerized records. These providers have agreed to make available their clients’ data to CDC researchers for the purpose of vaccine adverse event analysis and monitoring (as well as some other uses).47
Page 123
[…]
The CDC’s Vaccine Safety Office manages the VSD system.n 48 It is inaccessible to outside researchers, healthcare professionals, and the general public. Researchers who wish to analyze VSD data must submit a formal request to the CDC detailing their research proposal and intentions. Only CDC-approved researchers gain access to the system.49“
Chapter 3: Reference 47: “The Vaccine Safety Datalink: A Model for Monitoring Immunization Safety” James Baggs, 2011
Chapter 3: Footnote n: At the time of writing, Dr. Frank DeStefano is the head of the CDC Vaccine Safety Office. Remember that name for reference later in the book.
Chapter 3: Reference 48: Vaccine Safety Datalink
Chapter 3: Reference 49: Vaccine Safety Datalink (VSD)
“Ask you doctor:
Page 126
Are you familiar with the VAERS system? Have you ever filed a case with VAERS?
If your patient experiences an adverse health event following vaccination, do you check VAERS for reports of similar symptoms before deciding how to proceed with the case? Do you report it to VAERS?
Do you think healthcare professionals should be required by law to report adverse health events following vaccination, similar to their obligation to report cases of notifiable infectious diseases?“
Dr. Han provided a helpful correction or clarification in his third debunking article, claiming that:
“Under the National Childhood Vaccine Injury Act (NCVIA), healthcare providers are required by law to report to VAERS:
Dr. Frank Han: The Grand Debunk of the antivaxxer book “Turtles All the Way Down” (part 3/10)
– Any adverse event listed in the VAERS Table of Reportable Events Following Vaccination [PDF – 5 Pages] that occurs within the specified time period after vaccinations
– An adverse event listed by the vaccine manufacturer as a contraindication to further doses of the vaccine”
Factoring in Dr. Han’s criticism, perhaps the book should instead ask if doctors support expanding the range of adverse events they must report by law.
Chapter 4: Epidemiology 101
(20 pages, 29 references)
The fourth chapter is a helpful little one for anyone who is newer to these rabbit holes. It quickly explains the different types of studies, with their pros and cons.
“Epidemiological studies come in several varieties. In an interventional study (trial), subjects are asked by researchers to take specific actions (e.g., take a drug), while in an observational study, researchers only gather information about the subjects. A prospective study tracks a group of people for a specific period of time and collects relevant data about them. A retrospective study analyzes existing data.
Page 144
[…]
Despite the fact that formal techniques and statistical tools are used in epidemiological research, there are no fixed recipes to follow when designing studies. Researchers must carefully select the research method, collect complete and reliable data, neutralize any bias, and apply correct analytical methods. Producing high-quality, meaningful research is no simple matter; some even consider it an art form.”
Chapter 5: Purposely Biased Science: Epidemiology and Vaccine Safety
(54 pages, 114 references)
“Summary
Page 198
Epidemiological studies are the tool of choice for health authorities and pharma companies to maintain a facade of vaccine safety science. They are cheap, relatively simple to conduct, and, above all, their results are easily manipulated.
The five studies reviewed in this chapter illustrate some of the many methods researchers use to manipulate the results of epidemiological studies:
– Using unsubstantiated data (Madsen 2002).
– Using irrelevant data (Fombonne 2006).
– Hiding the real source of the data (Fombonne 2006).
– Omitting essential data from the paper (Grimaldi 2014).
– Reversing the trend of raw data by means of undisclosed statistical adjustments (Madsen 2002).
– Using arbitrary, meaningless, and scientifically baseless calculations (DeStefano 2013).
– Dismissing inconvenient findings on a speculative or arbitrary pretext (McKeever 2004).
– Misrepresenting the subject of the study to the public (DeStefano 2013).
– Using a grossly inadequate research method (Fombonne 2006).
– Using a research method that facilitates easy manipulation (DeStefano 2013, Grimaldi 2014).
– Failing to address post-publication misconduct allegations (Fombonne 2006).
– Overstating the significant of study results (all).
Amazingly enough, this assortment of faults did not prevent any of these studies from being published in leading medical journals or lead to their retraction. No mainstream scientist, academic, or journalist has directed a single critical word toward the studies or their authors. The studies’ scientific reputations remain unblemished to this day, and they are frequently cited in the medical literature and publications of health authorities as evidence of vaccine safety. None of those citing the studies ever mention their obvious flaws or the researchers’ conflicts of interest.”
“Ask you doctor:
Page 199
Do you know who funds most vaccine safety research? Are you familiar with the process used to allocate medical research grants?
Would you expect pharmaceutical companies and government agencies to fund vaccine safety studies that could potentially find serious faults in the vaccines they manufacture, license, and recommend to the public?
Are you aware that studies published in leading medical journals which ostensibly confirm the safety of vaccines suffer from serious methodological flaws and are fraught with authors’ conflicts of interest?“
FYI: In Dr. Han’s Turtles debunking article #5 he tried to take down the book’s claim that the Madsen 2002 study “actually show a 45% higher risk of autism in MMR vaccinated children, compared to the non-MMR-vaccinated” (Turtles page 166). Dr. Han does not provide a link to the actual study, but his screenshot also suffices:
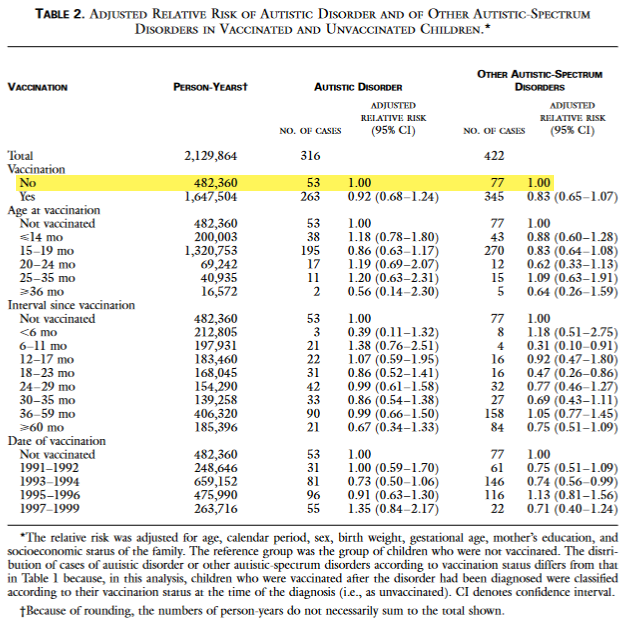
Dr. Han chides that “If the editors want to be seen as having any credibility at all analyzing studies, they shouldn’t be making such a simple mistake. […] The only pair of numbers up top that differ by 45% are the 53 and 77, which are numbers that delineate the number of children diagnosed with autism, versus the number of children with other autism spectrum disorders. Both were in the unvaccinated group.”
Since I am asking similar questions as the book’s authors, it took me one try, in one minute, to reproduce another 45% number derived from the raw data. First, I calculated the two rates of autistic disorder being compared:
Autistic Disorder Rate in Unvaccinated Children
= 53 / 482,360 = 0.000109876440833
Autistic Disorder Rate in Vaccinated Children
= 263 / 1,647,504 = 0.000159635424254Second, I calculate the percentage increase of the vaccinated rate over the unvaccinated rate:
( Rate in Vaccinated Children - Rate in Unvaccinated Children )
/ Rate in Unvaccinated Children
= 0.452863080053968
= 45.286%The Turtles book’s claim is correct that the raw data shows a 45% higher risk. Through the [more subjective] art of statistical analysis, this signal was buried. Dr. Han’s simple mistake is an example of failing to entertain skeptics’ questions with curiosity and humility. As usual, you can’t find what you don’t look for.
Clicking through links in Dr. Han’s piece, I did appreciate reading through one review article in Nature titled “Emerging concepts in the science of vaccine adjuvants” (Pulendran, Arunachalam, O’Hagan, 2021). I think this article provides interesting context and depth for those interested. The introduction mentions that “despite their widespread use, the molecular mechanisms by which the available adjuvants — including alum, MF59 and the Adjuvant Systems AS0 adjuvants — actually work in humans is not well understood.”
Chapter 6: The Studies That Will Never Be Done
(40 pages, 73 references)
This chapter covers the important sub-topic of the lack of safety testing of the entire childhood vaccine schedule as a whole. Studies comparing Vaccinated and Unvaccinated participants are often called “VU” studies.
“And what about the establishment itself? By now, you know that the medical establishment does not provide sufficient scientific evidence to support these safety claims, but you might be surprised to learn that government health agencies don’t actually make those guarantees. If you search the websites of the DHHS or the CDC for a statement claiming that the vaccination program of the United States has been tested for its effectiveness and safety, you will not find any. You may come across a webpage asserting that specific vaccines have been tested, individually or with other vaccines given at the same day according to the recommended schedule, or that vaccine adverse events are well monitored.30 But you will not find a clear and explicit statement arguing that the vaccine program as a whole has been properly tested and found to be safe and effective.
Page 217
In case you were wondering, this is not a coincidence or indicative of negligence on the part of the CDC or DHHS. Of course, it is easy to believe that a government body might be slightly negligent in making important information accessible to the public through its website. But this is not the current case. A formal statement attesting to the scientific validity of the vaccine program as a whole does not appear on health authorities’ websites because these institutions are well aware that it has never been tested. This fact was formally documented in the IOM 2013 report, which details many aspects of the vaccine program that have never been scientifically investigated.
Firstly, the report acknowledges that no studies have ever compared the overall health of fully vaccinated children to that of children who have never been vaccinated. In addition, no VU studies have been conducted on specific health outcomes such as autism or autoimmune diseases.31
[…]
Furthermore, when a new vaccine is added to the vaccine schedule, no studies are done to examine its effect on the other vaccines on the schedule. Research to evaluate different variations of the schedule, to ensure it is still ‘optimal’ is also never done (and bear in mind that each country implements its own vaccination schedule, with different vaccine products, doses, and timings, etc.).34“
Chapter 6, Reference 30: CDC web page ‘Vaccine Safety’ (archive, archive)
Chapter 6, Reference 31: IOM 2013 (P 5 (22))
Chapter 6, Reference 34: IOM 2013 (P 130 (147), P 31 (48))
“Ask you doctor:
Page 240
Are you familiar with any medical study that compared the overall health of vaccinated children to that of unvaccinated children?
In the absence of studies comparing the overall health of vaccinated vs. unvaccinated children, what is the scientific evidence for the safety and benefit of the vaccine program?
In the absence of a study comparing the overall health of children who vaccinated according to the official schedule to that of children who received no vaccines, would you still tell parents their children are better off getting all routine vaccinations? If so, on what grounds?“
The IOM 2013 Report
After reading chapter 6, I read the summary of the IOM 2013 report. It mentions that “With the current schedule, children may receive up to 24 immunizations by age 2 years and up to 5 injections in a single visit.” Some of the IOM 2013 summary’s conclusions include…
“CONCLUSIONS ABOUT SCIENTIFIC FINDINGS
The committee encountered two major issues in its review of the findings in the scientific literature. First, the concept of the immunization “schedule” is not well developed. Most vaccine-related research focuses on the outcomes of single immunizations or combinations of vaccines administered at a single visit. Although each new vaccine is evaluated in the context of the overall immunization schedule that existed at the time of review of that vaccine, elements of the schedule are not evaluated once it is adjusted to accommodate a new vaccine. Thus, key elements of the entire schedule—the number, frequency, timing, order, and age at administration of vaccines—have not been systematically examined in research studies.
The second major issue that the committee encountered was uncertainty over whether the scientific literature has addressed all health outcomes and safety concerns. The committee could not tell whether its list was complete or whether a more comprehensive system of surveillance might have been able to identify other outcomes of potential significance to vaccine safety. In addition, the conditions of concern to some stakeholders, such as immunologic, neurologic, and developmental problems, are illnesses and conditions for which etiologies, in general, are not well understood.Finally, the committee found that evidence assessing outcomes in sub-populations of children who may be potentially susceptible to adverse reactions to vaccines (such as children with a family history of autoimmune disease or allergies or children born prematurely) was limited and is characterized by uncertainty about the definition of populations of interest and definitions of exposures and outcomes.
IOM 2013, Page 11, Summary
In summary, to consider whether and how to study the safety and health outcomes of the entire childhood immunization schedule, the field needs valid and accepted metrics of the entire schedule (the “exposure”) and clearer definitions of health outcomes linked to stakeholder concerns (the “outcomes”) in rigorous research that will ensure validity and generalizability.”
To me and my personal risk-benefit-analysis-matrix, these admissions contradict the committee’s final conclusions:
“CONCLUDING OBSERVATIONS
IOM 2013, Page 14, Summary
The committee’s efforts to identify priorities for recommended research studies did not reveal an evidence base suggesting that the childhood immunization schedule is linked to autoimmune diseases, asthma, hypersensitivity, seizures, child developmental disorders, learning disorders or developmental disorders, or attention deficit or disruptive behavior disorders.
[…]
The committee found no significant evidence to imply that the recommended immunization schedule is not safe. Furthermore, existing surveillance and response systems have identified known adverse events associated with vaccination. The federal research infrastructure is a strong system.”
Chapter 7: Unsubstantiated Vaccination Guidelines
(26 pages, 31 references)
“It seems, therefore, that neither the WHO nor the CDC is aware of any timely and comprehensive safety assessment for the vaccine combinations currently recommended on the US childhood vaccine schedule. The most comprehensive review they cite, possibly the only one in existence, was published more than 25 years ago and does not cover most of the vaccine combinations given today. More up-to-date information doesn’t appear to be available. With no official record of the safety testing of current vaccine combinations, to determine what testing has actually been done, one must scan the medical literature for each of the individual combinations in use and find the studies performed for each of the individual products.
Page 246
Let’s consider, for example, the vaccines an American 15-month old might receive according to the schedule. According to the CDC,7 at 15 months a child could receive as many as 9 shots for 13 diseases.f (Different combinations of vaccine products exist for the applicable diseases, so the number of shots may vary between 5 and 9). A search of the medical literature failed to find any published report of a clinical trial, or even a retrospective study, which looked at the safety of concurrently administering this combination of vaccines.
[…]
Vaccinating with this approved combination of shots effectively enrolls American infants in a large-scale vaccine trial without the parents’ consent or awareness. Actually, using the term trial here is generous, as the results of this ‘experiment’ are not tabulated and will never be published.”
Chapter 7, Reference 7: Recommended Child and Adolescent Immunization Schedule, for ages 18 years or younger (P 2, table 1)
Chapter 7, Footnote f: The 13 diseases are hepatitis B, diphtheria, tetanus, pertussis, Hib, pneumococcal, polio, influenza, measles, mumps, rubella, varicella, and hepatitis A.
“Ask you doctor:
Page 261
Do you know of any studies that have examined the safety of the simultaneous administration of 9 vaccine injections against 13 different diseases to a 15-month year-old infant?
Are you familiar with any studies that have shown that spacing out vaccines does not reduce the number and severity of side effects?
Do you believe that administering 10,000 vaccines in one day to an infant is safe? If you do, can you provide studies that have demonstrated the safety of this procedure?
Do you know of any studies that examined the safety of the recommendation to vaccinate infants with mild illness? If not, what is the scientific evidence on which the CDC relies in determining that this would not increase the risk for vaccine injury?“
Part II: Founding Myths
Chapter 8: The Disappearance of Disease
(40 pages, 125 references)
I enjoyed this chapter as well, as it shed light on some interesting aspects of the history of sanitation, hygiene, etc…
“Following the publication of McKeown’s research, the academic consensus holds that the major contributors to the decline were improvements in sanitation and hygiene, a rise in the standard of living, better nutrition, and the gradual introduction of medical interventions during the 20th century.54“
Page 284
Chapter 8, Reference 54: McKeown 1972 (P 39)
“Life was harsh for urban horses, and average life expectancy was as low as four years. Thus, it was common in 19th-century cities for horses to collapse and die on the street. New York City, for example, cleared some 15,000 horse carcasses from the city’s streets in the year 1880 alone.69“
Page 287
Chapter 8, Reference 69: Tarr 1997 (P 2)
“The disparity between the societal burden of chronic and infectious disease morbidity has only grown wider from 1979 to the present. Infectious disease morbidity in children, which was already relatively low, continued to slowly decline. At the same time, the proportion of chronically ill and disabled children, which was already very high in 1979 (3.9%), more than doubled by 2010 (8%). In addition, it should be noted that for every disabled child in 2010, there were two others who routinely took medication for their chronic conditions.118 These numbers are considerably higher than, for example, the rate of children hospitalized due to an infectious disease during the same period (an average rate of about 1% in ages 0-19.).119“
Page 299
Chapter 8, Reference 118: More than 25% of Kids and Teens in the U.S. Take Prescriptions on a Regular Basis (archive)
Chapter 8, Reference 119: Infectious Disease Hospitalizations in the United States (P 3, Table 2) (archive)
Chapter 9: Herd Immunity
(62 pages, 253 references)
The end of chapter nine generously summarizes their 50~page analysis with hundreds of references and rabbit holes to explore…
“Routine Vaccines and Herd Immunity: A Summary
Page 358
Table 9-1 below presents the vaccines of the US childhood routine schedule and summarizes the relevant information regarding herd immunity for each. Of the 14 vaccines, only 5 (one-third) can be said to definitely provide relevant herd protection for children, as is detailed below.v 241“
Chapter 9, Footnote v: A similar list is provided by Dr. Stanley Plotkin, editor of the book Vaccines, in an article published in 2008 (see reference).
Chapter 9, Reference 241: Correlates of Vaccine-Induced Immunity (P 5)
| Disease | ||||
|---|---|---|---|---|
| Vaccine / Disease | Herd Immunity | Description | Pre-vaccine Incidence | Severity |
| Tetanus | – | The bacterium lives in animal feces and soil. The vaccine targets the toxin rather than the bacterium and therefore cannot prevent transmission. | Very rare | Lethal |
| Inactivated Polio (Salk) | – | The vaccine does not prevent replication of the virus in the gut and its excretion in the feces. | Low | Severe |
| Pertussis | – | The current (acellular) vaccine does not prevent infection with the bacterium or transmission to others. | High | Medium |
| Diphtheria | – | The vaccine works against the toxin, not the bacterium, and therefore does not prevent infection and transmission. There is no solid evidence for herd protection. | High | Severe |
| Influenza | – | Vaccine efficacy is moderate to low. The virus is constantly evolving; therefore a new vaccine must be formulated every year, targeting the strains that are expected to be common in the coming winter season. | High | Medium-high |
| Hepatitis A | – | The disease is very mild in children, and infection with the virus provides lifelong immunity. Children receive no benefit from the herd protection provided by the vaccine. | Low | Very mild in children (moderate in adults) |
| Hepatitis B | – | The vaccine is redundant for about 99% of children. The herd protection it provides is relevant only to children living with a chronic carrier of the disease. | Low | Moderate |
| Rotavirus | – | The disease itself does not fully prevent future infection or morbidity. There is some evidence for the existence of a herd protection effect for the vaccine, but the body of research on the subject is preliminary and definitive conclusions cannot be drawn. | Very high | Mild-moderate |
| Pneumococcal | +/- | The vaccine provides partial herd protection – only against the serotypes it contains. It does not reduce the rate of bacterial carriers in the population due to the phenomenon of serotype replacement. | Low | Moderate |
| Hib | + | There is epidemiological and biological evidence of vaccine-induced herd immunity. | Low | Moderate |
| Varicella (Chickenpox) | + | The vaccine appears to provide herd protection, but it is not routinely given in some industrialized countries. | Very high | Mild |
| Rubella | + | The vaccine appears to have nearly extinguished the spread of the virus in the population. | Low | Very mild in children (serious in fetuses) |
| Mumps | + | The vaccine almost completely eliminated morbidity (despite sporadic outbreaks in adults reported from time to time). | High | Mild |
| Measles | + | The vaccine almost completely eliminated the disease. | Very high | Mild |
Chapter 10: The Mysteries of Polio
(128 pages, 447 references)
This monster of a chapter dives deep into the history of polio science. Most of this was new to me. But I thought the authors make compelling arguments pointing to very weak foundations.
“Although the amount of spray residue left on fruit marketed to American consumers steadily increased from the end of the 19th century onward,202 federal authorities made no effort in the early 20th century to control or limit the amount of arsenic or lead in agricultural produce.203 It was only in the 1920s that the Bureau of Chemistry at the US Department of Agriculture began testing arsenic levels in fruit and vegetable shipments. […] Unfortunately, the inherent conflict of interest between the Bureau of Chemistry’s role (overseeing the use of pesticides in agriculture) and its Department’s stated mission (promoting agriculture) limited the Bureau’s actions and hampered its ability to enforce its own recommendations.204 Attempts to impose maximum thresholds for lead and arsenic content in fruit met, as expected, with fierce opposition from farmer organizations and elected officials who acted on their behalf.205“
Page 426
Chapter 10, Reference 202: Before Silent Spring: Pesticides and Public Health in Pre-DDT America (James Whorton, 1974, P 71)
Chapter 10, Reference 203: (James Whorton, 1974, P 69)
Chapter 10, Reference 204: DDT – Scientists, Citizens and Public Policy (Thomas Dunlap, 1981, P 42)
Chapter 10, Reference 205: (James Whorton, 1974, Chapters 4-5)
“By the time the veil concealing the dangers of toxic pesticides was lifted in the 1930s and papers demonstrating pesticides’ harms began appearing in medical journals, the virus theory of polio was already well established. Thus, the odds that a scientist or a doctor would look for or recognize a link between lead arsenate and paralytic polio was quite slim. A polio researcher who went against the established dogma was liable to be sharply criticized by his peers and ignored by the National Foundation for Infantile Paralysis, which in those days sponsored virtually all polio research. Moreover, this hypothetical maverick researcher would likely have been the object of brutal attacks by the agricultural lobby and the big pesticide-producing chemical companies.bb“
Page 428
Chapter 10, Footnote bb: A similar attack was mounted against Dr. James Putnam and his colleagues, the Boston doctors who in the late-19th century put forth the health harms of the domestic use of Paris green. (Whorton 1974, 58-59).
“After four years of meticulous research, Rachel Carson published Silent Spring in 1962, publicly exposing the harmful environmental effects of DDT for the first time. Carson’s book raised awareness of DDT’s impact on wildlife instantaneously, initiating public debate and a legacy of environmental activism that lasts to this day. While the destructive effects of DDT were new to the public, government authorities, however, had no need for such an introduction. They had been aware of the compound’s dangers for years and, as we shall soon see, had already taken steps to monitor, and even reduce, its use.
Page 474
As early as 1946 Dr. Fred Bishopp, from the Bureau of Entomology of the US Department of Agriculture, was writing about the toxicity of DDT to the nervous system.395 In a paper published in the American Journal of Public Health, Bishopp warns that ‘DDT must not be allowed to get into foods’396 and care must be taken when spraying it on crops intended for human or animal consumption.397“
Chapter 10, Reference 395, 396, 397: Present Position of DDT in the Control of Insects of Medical Importance (Fred C. Bishopp, 1946, P 2)
Born near the start of the 80s, I barely remember the environmental movement before it was coopted by ‘climate change’ alarmism (based on computer models and a non-falsifiable hypotheses). Since the early 2010s, I have concluded that it would be far more help to our environment if we focused on all the man-made novel pollutants which are intended or known to harm life… instead of carbon which sustains life.
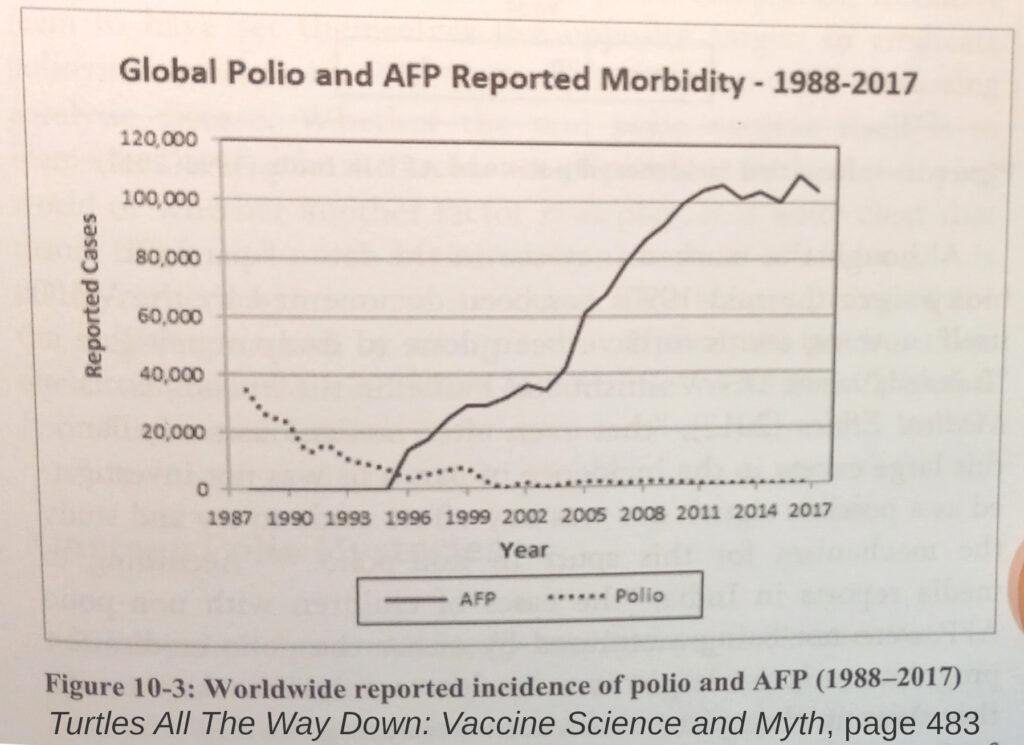
“Over time, more and more cases of non-polio flaccid paralysis were identified and placed under the newly formed umbrella of acute flaccid paralysis (AFP) syndrome.
Page 482
[…]
While reported polio morbidity dropped to almost zero between 1988 and 2010, the global incidence of AFP has risen to about 100,000 cases in 2010, three times the global level of paralytic illness recorded in 1988.441“
Chapter 10, Reference 441: Plotkin 2013 – Vaccines (6th edition, P 638, picture 28-12)
“Nineteen Polio Mysteries
Page 486
[…]
The list below summarizes the polio ‘mysteries’ discussed in this chapter. For each, we present explanations (if any) according to both the officially sanctioned history of polio and the Pesticides Theory.
1) Why did polio epidemics emerge in the United States specifically in the late-19th century?
Institutional: Due to better hygiene, first exposure to the poliovirus occurred later in life, often when people no longer had protective maternal antibodies. This is the Improved Hygiene theory, which as the chapter shows, is inconsistent with the biological evidence and flatly contradicts most of the epidemiological evidence as well.
Pesticides: Pesticides (particularly lead arsenate) began to be widely used in the US in the late-19th century.
2) Why did polio epidemics strike industrialized countries in the first half of the 20th century, when almost none occurred in the developing world?
Institutional: According to the (senseless) Improved Hygiene theory, in the Third World sanitation and hygiene remained poor, so polio incidence also remained very low.
Pesticides: Before WWII, pesticides were rarely used by indigenous people in developing countries.
3) Why did polio hit hardest in the summer and early autumn?
Institutional: No explanation.
Pesticides: In the northern hemisphere, the majority of (sprayed) fruits and vegetables were picked and consumed in the summer and autumn months.
4) Why did polio’s incidence peak in the summer, when most children are not attending school, even though the disease’s purported mode of transmission (person to person) favors crowded conditions?
Institutional: No explanation.
Pesticides: Person-to-person transmission is largely irrelevant for pesticide-induced morbidity.
5) Why did most of the early polio outbreaks occur in sparsely populated rural areas rather than in the large and crowded metropolitan areas?
Institutional: No explanation.
Pesticides: Farming communities were the first to be poisoned by the use of toxic pesticides.
6) Why were many of the early polio outbreaks in rural areas accompanied by concurrent outbreaks of paralysis in domestic animals?
Institutional: No explanation.
Pesticides: Farm animals and pets, like humans, were also exposed to pesticides in their food and environment.
7) Why did polio emerge and intensify during a historic period when mortality and morbidity of most infectious diseases were dramatically declining?
Institutional: Polio emerged due to better sanitation and hygiene (while other diseases declined for the same reason).
Pesticides: Paralysis epidemics weren’t caused by an infectious agent but rather by pesticide intoxication. Pesticide use increased during this period.
8) Why were high polio rates observed in European residents of developing countries, while local residents rarely experienced acute flaccid paralysis?
Institutional: According to the Improved Hygiene theory, Europeans were not exposed to the poliovirus as infants and got sick when first exposed to the virus (which was ubiquitous in developing countries) at an older age.
Pesticides: European residents in developing countries were protecting themselves from local insects with pesticides that the locals didn’t use.
9) Why was polio incidence much higher in Western soldiers stationed in different parts of the world during and after World War II than in their comrades back home?
Institutional: According to the (senseless) Improved Hygiene theory, these soldiers were more exposed to poliovirus in foreign countries than their peers at home.
Pesticides: The overseas military environment was heavily sprayed with pesticides to fight disease-carrying insects.
10) Why was polio incidence among WWII British officers in India, North Africa, and Italy five to ten times higher than that of British enlisted men?
Institutional: No explanation.
Pesticides: Officers’ barracks, clubs, etc., were presumably sprayed more extensively, to protect the officers even more than enlisted men.
11) Why were polio’s disease patterns (for example, the age distribution of patients being similar in epidemic and low-prevalence years) so different for those typical of other childhood infectious diseases?
Institutional: No explanation.
Pesticides: Epidemics were dependent on various factors related to pesticide use.
12) Why did polio incidence suddenly skyrocket in many industrialized nations after World War II?
Institutional: No explanation.
Pesticides: Many countries which had not used pesticides much prior to WWII began using DDT extensively in the post-war years due to its safety reputation, low price, and high efficacy.
13) Why did polio epidemics in post-WWII United States begin occurring in consecutive years, rather than once every few years as in the pre-war period?
Institutional: No explanation.
Pesticides: DDT use in the post-war years far exceeded that of the pesticides in the pre-war era.
14) Why, contrary to other infectious diseases, are polio patients virtually non-contagious, while the disease is presumably spread so easily by healthy people?
Institutional: No explanation.
Pesticides: Morbidity wasn’t due to an infectious agent, so these questions are irrelevant.
15) Why do several members of the same household tend to contract polio at the same time, rather than one after the other, as usually happens with infectious diseases?
Institutional: No explanation.
Pesticides: Morbidity wasn’t due to an infectious agent. Several members of a household could easily be simultaneously exposed to toxic compounds from food or other sources, such as spraying devices in the home.
16) How did the Salk vaccine manage to nearly eradicate polio in the US (and other countries), even though at least 20% of the paralysis was not caused by the poliovirus?
Institutional: No explanation.
Pesticides: Polio-like morbidity was mainly caused by exposure to pesticides. The drop in morbidity started before the Salk vaccine and continued in all segments of the population, regardless of the vaccine.
17) How did the Salk vaccine manage to completely eliminate the spread of the poliovirus in countries where it was given exclusively, even though the vaccine essentially does not confer herd immunity?
Institutional: No explanation.
Pesticides: Same as above.
18) Why didn’t polio epidemics appear in the developing world until the second half of the 20th century?
Institutional: Polio thrives in conditions of poor sanitation and hygiene that are prevalent in the developing world (never mind the fact that this contradicts both the Improved Hygiene theory and epidemiological evidence from the earliest polio outbreaks).
Pesticides: After WWII, the use of pesticides in developing countries expanded rapidly, especially DDT and lead-and arsenic-based compounds.
19) Why is polio in the developing world associated with a poor standard of living, while in the West it was linked to the opposite?
Institutional: No explanation.
Pesticides: Polio-like paralysis is mainly caused by pesticide use and has little to do with standards of living (in the broad sense).”
“The foundations of polio science are so inadequate and sketchy, despite more than a hundred years of intensive research effort, that it’s small wonder it does not provide convincing solutions to most of the mysteries and question marks surrounding the disease since it became a public health threat in the late-19th century.”
Page 494
I never would have guessed the history of polio science that this book presents. And I look forward to learning more about this sub-topic in the future.
Chapter 11: The Vaccine Hoax
(21 pages, 0 references)
The final chapter provides a succinct summary of each previous chapter, followed by a discussion of the information wars.
“The last person to attempt injecting some vaccine truth was talk-show host Katie Couric, who in 2013 interviewed a mother whose daughter died shortly after receiving the HPV vaccine, Gardasil. Following the airing of the show, Couric was immediately attacked by every major news outlet for, in her own words, ‘[spending] too much time on the serious adverse events that have been reported in very rare cases following the vaccine.’ She was quick to issue a public apology, thus, presumably, paying the price for her ‘dire mistake’ and saving her career. To date, no one in mainstream media has dared to follow in her footsteps.”
Page 514
“A 21st century debate, conducted on an internet-based platform such as Facebook, would be the perfect setting to allow a comprehensive, thorough, and productive debate. The two sides could lay down their arguments meticulously, providing references and supporting material as required. The debate could go on for days, or even weeks, elucidating relevant topics as the need arose. The audience could follow at their leisure, weigh the arguments made by both sides, check the references, and make up their own minds. If one side played unfairly — evading questions, refusing to back up their arguments, etc. — this would be noted by the viewers and reflected in their success.
Page 516
Such a debate would be the perfect mechanism for settling many of the controversies surrounding vaccination. And that is exactly why the medical establishment’s spokespersons will NEVER engage in such a debate, wherein substance would speak louder than sound bites. Just imagine a debate where the vaccine ‘experts’ are publicly asked the inconvenient questions this book has presented. They would have no choice but to evade direct replies as they have no adequate answers. Within days, this debate could be viewed by millions all over the globe, spelling a PR mega-disaster for the vaccine establishment.”
And the book’s concluding thoughts resonate deeply with me. They echo logic and philosophies that I have also tried to promote over the years…
“Science is ever evolving, and free scientific discussion guarantees its progress. True scientists are not afraid of discussion — they are eager for it. A one-sided scientific discussion is a feature of dark historical periods and totalitarians regimes, not free democratic societies.
Page 518
Science belongs to the people. It belongs to humanity, not to corrupt government agencies and pharmaceutical giants who collude to rewrite the principles of science in order to continue the decades-long cover-up of their crimes against humanity.
The magnitude of these crimes is enormous — these entities are in way too deep to ever be able to admit any wrongdoing. They will do whatever is necessary to protect the great vaccine hoax. For them, it is a matter of life and death — literally.
And so it is for us.”
My Conclusions
This book presents dozens of critical questions which it alleges still demand answers. It is definitely worth reading and considering. These questions warrant further investigation, transparent data, and nuanced communication from health authorities.
I made some efforts to try to find attempts to debunk or refute the key claims made in this book. I found a few articles and discussed them above, but they did not seem to directly address or refute key claims. The vaccine evangelists’ rebuttals were almost always addressed or refuted within the book.
Most of all, it seems that critics of this book simply agree with the current ‘ethical’ paradigm for vaccine trials. This paradigm is endorsed by the health authorities intertwined with the pharmaceutical industry.
During the 2009 Swine flu ‘pandemic’, I was skeptical or terrified of the vaccine which was quickly released for such a ‘novel’ and ‘deadly’ virus. This episode is when I got interested in the biosecurity state and medical martial law. I was also listening more seriously to parents and doctors who were trying to sound the alarm on childhood vaccine schedules.
I was very opposed to almost all fear-mongering and public policy reactions to the COVID-19 ‘pandemic’ from its start in early 2020. Millions of us were asking critical questions throughout the pandemic. Scientists investigating COVID’s Infection Fatality Rate (IFR) published that “The median IFR was 0.0003% at 0–19 years”. (Ioannidis, Jan 2023) The idea of vaccinating children with the new experimental COVID injections never passed any kind of risk-benefit analysis.
Published in the British Medical Journal in 2021, “On paper, the phase III studies by Pfizer, Moderna, and Janssen are all of two years’ duration.” But after collecting only six months of data on efficacy and safety, these received Emergency Use Authorization (EUA). And “within weeks of the vaccines receiving an EUA the unblinding of trials commenced as placebo recipients were offered the chance to get vaccinated.”
Operation Warp Speed was brought to you by Donald Trump, Moncef Slaoui, Anthony Fauci, and an army of others. They never included tests for the shots’ effects on virus transmission. Between the low IFR estimates, the lack of long-term clinical safety data, and no claims of [significant] reduction in transmission, I do not think it was ever ethical to give COVID injections to children at all.
Any doctor or health authority that recommended experimental COVID injections for children completely lost my trust [at least regarding some issues].
The CDC’s vaccine schedule recommends children receive COVID-19 gene therapy shots starting at six months old. Since the clinical trials were unblinded, that is the same age as the safety data they rely upon.
Over the past four years, countless millions of Americans are finally exploring related rabbit holes of alternative data. The ‘skeptics’ got more organized in the face of public policy emergencies. These policies violate basic liberties, and to varying degrees, bodily autonomy.
My strong opposition to the COVID shots has long felt very informed. But I now have a baby and needed to know more about childhood vaccines. So this book, Turtles All The Way Down: Vaccine Science and Myth, has been very helpful in working through the various sub-topics and debates. It identifies important dividing lines between various narratives and bodies of evidence. And I look forward to finding or hearing some more answers to some of the critical questions posed here.
If a fraction of this book’s conclusions are factually accurate, then we face a bleak situation and the tide is still turning very slowly. I also recently read another book, Vax-Unvax: Let The Science Speak, by Robert F. Kennedy Jr. and Brian Hooker, PhD, from 2023. It concludes with six constructive ideas, which the book describes in more detail.
So I will leave you with these proposed solutions which may be able to transcend partisan politics and politicized sciences. As you build your own risk analyses and conclusions, be sure to search out a variety of informed viewpoints, and encourage open debate. I wish you luck diving into these topics, as they are tough but critical. (Wish me luck, too!)
“The Vaccine Safety Project of Children’s Health Defense [CHD] is an outcome of an investigative review of the US government’s vaccine approval/recommendations process and post-marketing safety monitoring. CHD and Robert F. Kennedy Jr. formulated these six steps as necessary recommendations for improving vaccine safety and protecting children from vaccine injuries.
Vax-Unvax, Page 184
…
6 Steps to Vaccine Safety
1. Subject vaccines to the same rigorous approval process as other drugs.
2. Mandatory reporting of vaccine adverse events and automate the VAERS* and VSD* databases.
3. Ensure everyone involved with federal vaccine approvals and recommendations are free from conflicts of interest.
4. Reevaluate all vaccines recommended by the ACIP* prior to the adoption of evidence-based guidelines.
5. Study what makes some individuals more susceptible to vaccine injury.
6. Support fully informed consent and individual rights to refuse vaccination.
*VAERS: Vaccine Adverse Events Reporting System, *VSD: Vaccine Safety Datalink, *ACIP: Advisory Committee on Immunization Practices”
Feedback, comments, and answers to this post’ questions are welcomed via Wiki World Order’s Minds profile.
Updates & Corrections: The section within ‘debunking attempts’ for polio have been corrected to reflect that Professor Reiss was focused on the Hep B vaccine, not the Hib vaccine. I was astonished that none of her supporting references backed up her claims, and erroneously blurred the two vaccines in my discussion.
Within the discussion of the 45% risk increase in Madsen 2002, this article initially included a typo. In the blog post, my ‘second calculation’ had a division sign instead of a minus sign, in the numerator. This error was only in the blog post, and not the calculations reported here.
Morgan Lesko is a software engineer who wants collective decisions to be more evidence-based, especially wherever informed consent is likely to be violated. He only has a B.S. from the University of Maryland in 2005 for Computer Science with a concentration in Mathematics. He does not claim this is a rigorous scientific inquiry. This is just a thoughtful book review.
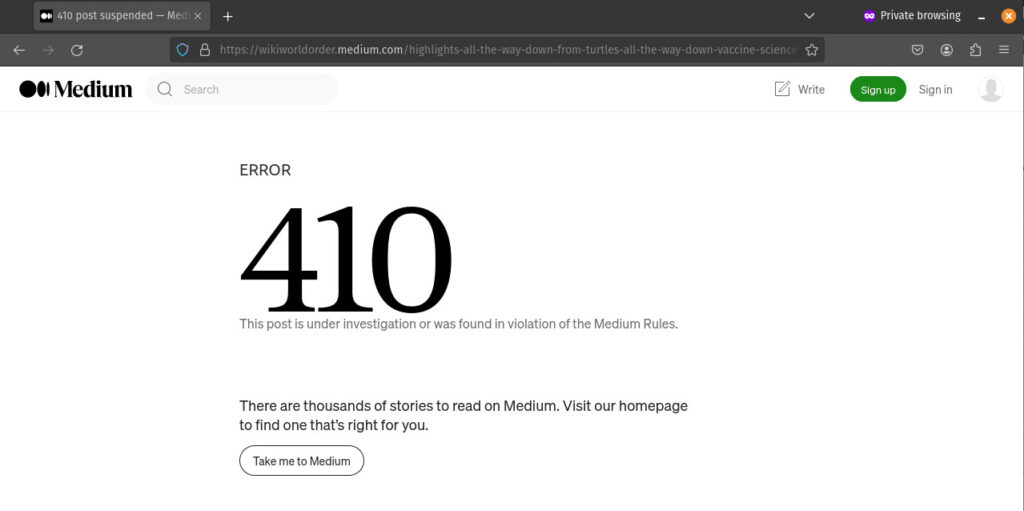
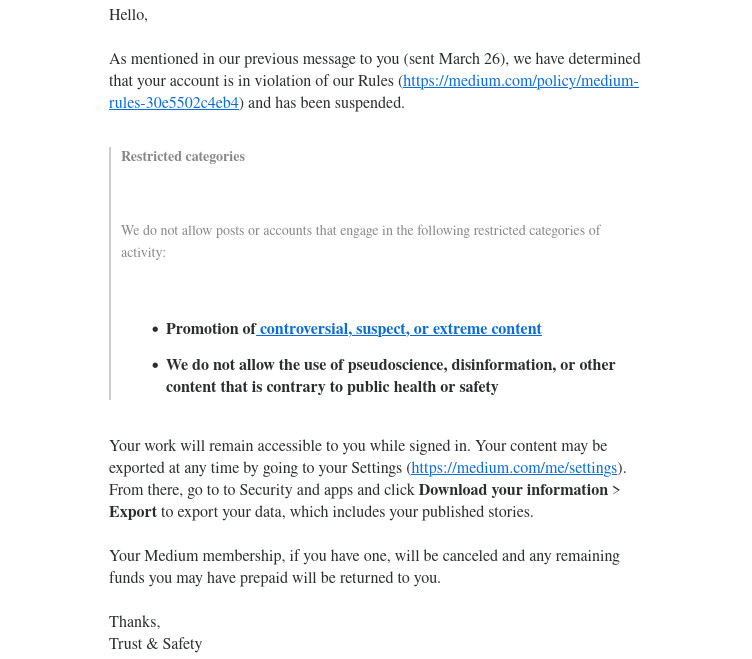
This article was cross-posted on Medium on 3/21/24. But it caused my account to be suspended on 3/26/24:
Related Content by Wiki World Order (WWO)
Double-Edges of Exponential Scientism 3/29/20
Compartmentalized Conspiracies: Would Anyone Have Talked? 12/18/19
Please Compartmentalize “the Viability of Conspiratorial Beliefs” 8/24/16
